Product Name: Fda cardiomyopathy outlet
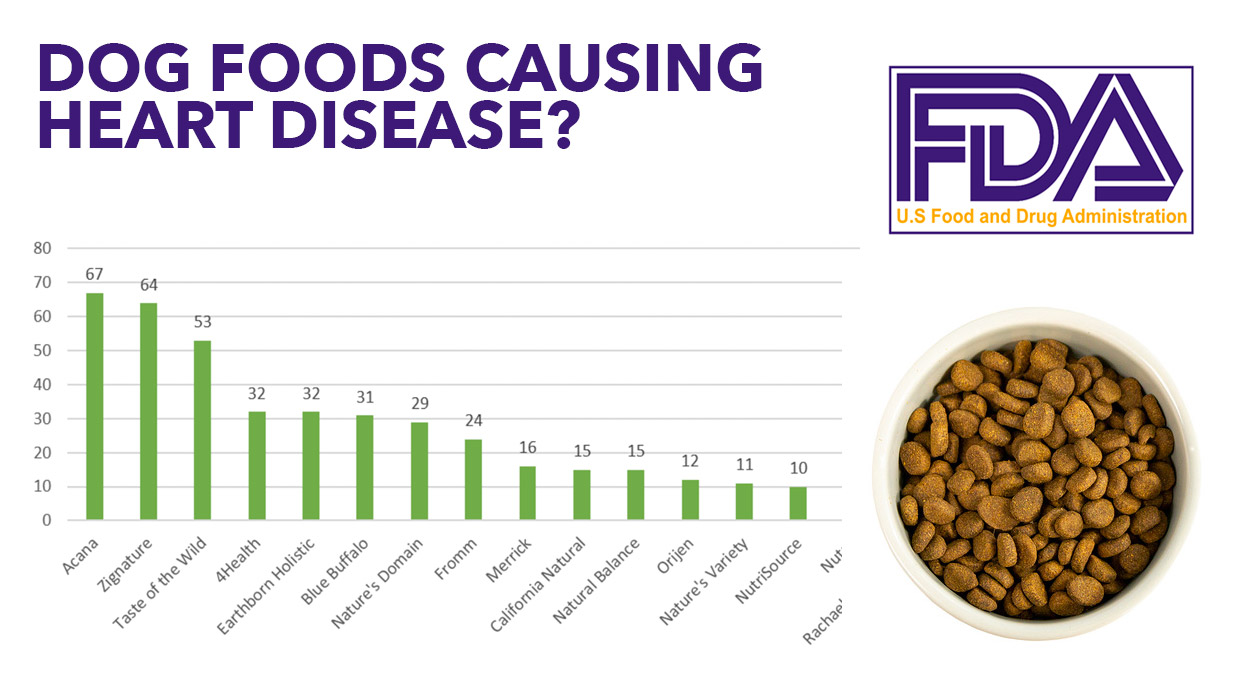
FDA Investigation into Potential Link between Certain Diets and Canine Dilated Cardiomyopathy FDA outlet, FDA Clears New Treatments for Cardiomyopathy Caused by Transthyretin Mediated Amyloidosis DAIC outlet, FDA Approves Drug for Hypertrophic Cardiomyopathy Health Hive outlet, FDA Investigation into Potential Link between Certain Diets and Canine Dilated Cardiomyopathy FDA outlet, FDA approves mavacamten for obstructive hypertrophic cardiomyopathy outlet, FDA Panel Reluctantly Backs Patisiran for ATTR Cardiomyopathy MedPage Today outlet, FDA Approves First Anti Inflammatory Drug for Heart Attack and Stroke Prevention outlet, Tenaya Therapeutics Receives Orphan Drug Designation from the U.S. Food and Drug Administration for its Gene Therapy for Genetic Arrhythmogenic Right Ventricular Cardiomyopathy DAIC outlet, FDA Investigation into Potential Link between Certain Diets and Canine Dilated Cardiomyopathy FDA outlet, Dilated Cardiomyopathy is. I Love Veterinary Medicine Facebook outlet, Food for Thought Updated outlet, Until more science is available FDA will end public updates on potential link between certain diets and canine dilated cardiomyopathy American Veterinary Medical Association outlet, FDA Investigation into Potential Link between Certain Diets and Canine Dilated Cardiomyopathy FDA outlet, Cardiomyopathy in Duchenne outlet, FDA Approves Mavcamten for the Treatment of Obstructive Hypertrophic Cardiomyopathy Docwire News outlet, FDA clears AI model for detecting signs of heart failure in ECGs outlet, FDA Issues List of Foods Linked to Dilated Cardiomyopathy Amelia Grace Animal Hospital outlet, FDA Warns Epinephrine Use Can Lead To Stress Cardiomyopathy outlet, FDA Update on Dilated Cardiomyopathy in Dogs. How Helpful was the FDA Update on Diet related Dog Heart Disease outlet, FDA Investigation into Potential Link between Certain Diets and Canine Dilated Cardiomyopathy FDA outlet, Heart Failure Drug Development CFR Journal outlet, U.S. Food and Drug Administration The FDA is investigating the potential association between reports of heart disease canine dilated cardiomyopathy DCM and certain pet foods the animals consumed conta... outlet, Viz.ai Receives FDA De Novo Approval for Hypertrophic Cardiomyopathy AI Algorithm G MedTech News Center outlet, Fda Dog Food Heart Disease 2024 kalamaja.ee outlet, How to Protect Your Dog s Heart Understanding Dilated Cardiomyopathy and Prevention Tips outlet, Viz.ai Receives First De Novo Approval by the FDA for Hypertrophic Cardiomyopathy AI Algorithm DAIC outlet, FDA Investigation into Potential Link between Certain Diets and Canine Dilated Cardiomyopathy FDA outlet, Dog food brands most linked to heart disease reports named News VIN outlet, Understanding the FDA Update on Non Hereditary Dilated Cardiomyopathy DCM in Dogs The Animal Medical Center outlet, Tenaya Therapeutics Announces FDA Clearance to Begin Clinical Testing of TN 401 Gene Therapy for the Treatment of PKP2 Associated Arrhythmogenic Right Ventricular Cardiomyopathy DAIC outlet, Eko Health Gains FDA Clearance For Cardiology Algorithm That Can Detect Heart Failure in 15 Seconds MedCity News outlet, Interventional treatment for heart failure receives FDA approval BIBA Medtech Insights outlet, Viz.ai poised to take a bite out of hypertrophic cardiomyopathy with FDA de novo BioWorld outlet, Restore Medical wins FDA breakthrough nod for heart failure device outlet, Fda Dog Food Heart Disease 2024 kalamaja.ee outlet.
Fda cardiomyopathy outlet